Practice Essentials
Human dirofilariasis typically manifests as either subcutaneous nodules [1] or lung parenchymal disease, in many cases asymptomatically. The zoonotic filariae of Dirofilaria immitis and Dirofilaria (Nochtiella) repens are increasingly recognized worldwide as inadvertent human pathogens, [2, 3] with the usual hosts of these infective nematodes being domestic and wild carnivores. [4]
Signs and symptoms
D immitis
Most patients (approximately 60%) with human pulmonary dirofilariasis (HPD), caused by D immitis, are asymptomatic. [2] When they do occur, symptoms can include the following [3] :
-
Localized retrosternal chest pain
-
Cough
-
Hemoptysis
-
Wheezing
-
Low-grade fever
-
Chills
-
Malaise
D repens
Usually, patients notice a single painful subcutaneous lump in the affected area. [1] The areas most commonly affected include the following [3] :
-
Face and eyelids
-
Chest wall
-
Upper arms
-
Thighs
-
Abdominal wall
-
Male genitalia
Ophthalmic involvement may be periorbital, [5, 6] subconjunctival, [7, 8] subtenon, [9] or intraocular. [3, 10]
See Clinical Presentation for more detail.
Diagnosis
Studies used in the diagnosis and evaluation of dirofilariasis include the following:
-
Complete blood count (CBC) - Using a CBC, eosinophilia may be detected in up to 20% of cases of HPD
-
Sputum cytology - The presence of eosinophils may support a diagnosis of HPD in patients with a coin lesion observed on radiography, although the test lacks enough specificity for an accurate diagnosis
-
Serologic studies - Using ELISA, serologic studies may yield positive results in 75% of patients with HPD
-
Polymerase chain reaction (PCR) assay - Has been successful in the diagnosis of D immitis and D repens infections
-
Imaging studies - Including chest radiography, computed tomography (CT) scanning, magnetic resonance imaging (MRI), and ultrasonography
-
Pulsed-field gel electrophoresis
-
Biopsy - Including surgical biopsy and fine-needle aspiration
-
Histology - Diagnosing dirofilariasis based purely on histopathology has its pitfalls, especially when the morphology of the nematode is altered owing to inflammatory response or surgical artifact
See Workup for more detail.
Management
Antifilarial medications
In many cases, antifilarial medications are not administered prior to surgical resection of dirofilarial lesions. One group of authors, however, has recommended a single dose of ivermectin followed by 3 doses of diethylcarbamazine (DEC) if the syndrome is strongly suspected prior to surgery.
Surgery
Surgical excision of lesions and affected areas is the treatment of choice for dirofilariasis. [11] Patients with subcutaneous dirofilarial lesions usually can be treated as outpatients or undergo day-case surgical procedures.
See Treatment and Medication for more detail.
Background
Human dirofilariasis typically manifests as either subcutaneous nodules or lung parenchymal disease, in many cases asymptomatically. (See Pathophysiology and Clinical Presentation.)
The zoonotic filariae of Dirofilaria immitis and D (Nochtiella) repens have become increasingly recognized worldwide as inadvertent human pathogens, [3, 4] with the usual hosts of these infective nematodes being domestic and wild carnivores. The pathology of dirofilariasis results from the aberrant localization of immature worms intended for nonhuman hosts. The worms do not reach adulthood in humans, which is why microfilariae are almost always absent (see the image below). (See Pathophysiology, Etiology, and Workup.)
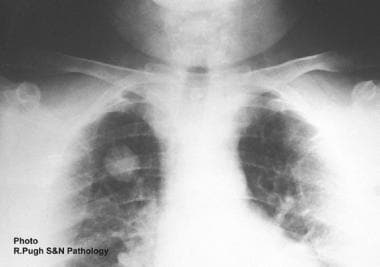 Plain chest radiographic appearance of pulmonary coin lesion secondary to Dirofilaria immitis infection in a man.
Plain chest radiographic appearance of pulmonary coin lesion secondary to Dirofilaria immitis infection in a man.
Dirofilaria, from the Latin dirus (“fearful” or “ominous”) plus filum (“thread”), is a genus of nematodes of the superfamily Filarioidea. The dog heartworm was named Filaria by American parasitologist Joseph Leidy in 1856, and the genus was renamed Dirofilaria by French parasitologists Railliet and Henry in 1911. [12]
Patient education
Teaching patients to avoid mosquito bites in endemic areas may decrease exposure to infection. (See Treatment.)
Pathophysiology
The dirofilarial life cycle, like that of all filarial and helminthic nematodes, consists of 5 developmental or larval stages in a vertebral host, an arthropod (mosquito) intermediate host, and a vector. Adult female worms produce thousands of first-stage larvae (microfilariae), which are ingested by a feeding insect vector. After ingestion, microfilariae eventually transform into third-stage larvae within the mosquito and migrate from the abdomen to the thorax and finally to the salivary glands, allowing transmission of infection to a new host upon a subsequent blood meal. [13]
The usual definitive host of D immitis and D repens is the domestic dog, although cats, wolves, coyotes, foxes, muskrats, and sea lions may act as suitable hosts and reservoirs of the disease. Mosquitoes of the genera Aedes, Anopheles, and Culex all are suitable intermediate hosts and vectors. Some species of fleas, lice, and ticks may also act as vectors. (See the image below.)
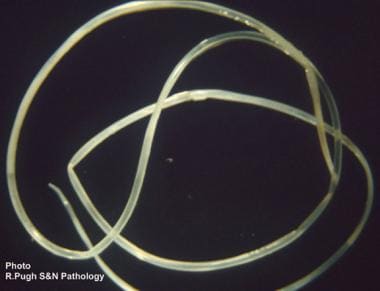 Adult Dirofilaria immitis worm extracted from the heart and pulmonary artery of a dog. Worms usually are 1-3 mm in diameter, and females may grow to as long as 30 cm.
Adult Dirofilaria immitis worm extracted from the heart and pulmonary artery of a dog. Worms usually are 1-3 mm in diameter, and females may grow to as long as 30 cm.
In the dog, adult D immitis resides in the right ventricle and pulmonary arteries of the lung. Female worms produce and release thousands of microfilariae larvae into the circulation daily, which are ingested by mosquitoes in a blood meal. Larvae develop into infective larvae over the subsequent 10-16 days, depending on environmental conditions, before being reintroduced back into the dog. These larvae reside and mature in the subcutaneous tissues and along muscle sheaths for the next several months before migrating to the heart, where the nematode matures for 6-7 months. Adult worms are 1-3 mm in diameter, with females being 25-30 cm in length and males generally shorter.
The adults of D repens, in contrast, reside in the subcutaneous tissues of dogs and cats, although the life cycle and release of microfilaria in the peripheral circulation remain the same as for D immitis. [14]
Human hosts
Human hosts are infected in the same way that the natural animal hosts are. Humans are accidental, dead-end hosts of dirofilariae, as the worms do not reach maturity in the heart or skin. Most infective larvae injected into humans are thought to perish; therefore, infected individuals are normally not microfilaremic.
Indeed, only 1 degenerate, immature (fourth-stage) larva or adult worm (fifth-stage larva) is usually isolated from an ectopic position of the body. Moreover, dirofilariae, like other nematodes, rely on sexual reproduction before microfilariae are produced. Only 1 report of circulating microfilaremia in a human exists in the medical literature. [15]
D immitis infection
The true prevalence of human D immitis exposure and disease may be underestimated, because canine infection is widespread throughout the world and most infected people are asymptomatic. A study of D immitis infections in endemic areas of northeastern Italy indicated that the mosquito host Culex pipiens fed on dogs 70% of the time and on humans 26% of the time. [16]
D immitis is most often associated with coin lesions of the lung (human pulmonary dirofilariasis, or HPD), but isolated reports exist of D immitis or D immitis –like worms causing cutaneous or conjunctival disease.
Extrapulmonary D immitis infections described in the literature include a hepatic nodule, [17] eosinophilic meningitis, [18] intraocular infection, [19] adipose tissue of the mesentery, [20] and a testicular lesion. [21]
Acute thromboembolism and endarteritis can result from exposure of vascular endothelium to a high concentration of D immitis antigenic products, caused by the sudden death of a significant number of adult worms; this is true whether or not the worms died naturally or were killed by a filaricide treatment. [22]
To date, only 4 cases of cardiovascular dirofilariasis have been reported worldwide. In the first case, D immitis (male and female) was found in the left ventricle of a Brazilian boy (Magelhaes, 1887). [23] Another case was in Japan, where Takeuchi et al [24] found 2 slender nematodes in the heart and inferior vena cava of a 36-year-old Japanese man who died of liver cirrhosis. Both worms were identified as nongravid adult females of D immitis. The other 2 cases, both involving D immitis, were reported in New Orleans in the United States. [23]
Takayama et al described a rare case of cavity formation in pulmonary dirofilariasis in a Japanese woman infected with D immitis. [25] A subcutaneous or subconjunctival lesion due to D immitis is also rare.
D repens infection
D repens infection is the most frequent and widespread form of dirofilariasis in the world. The most common form is a subcutaneous or submucosal nodule. [26]
Ophthalmic involvement is also described, and the worm can be directly visualized in the bulbar conjunctiva.
Breast nodules due to infection with D repens, which are observed in hyperendemic areas for the parasite, usually Italy and Sri Lanka, may be misdiagnosed as potential malignancies. Imported cases of D repens affecting the breast have been reported in the United States, Canada, Japan, and Australia, while pulmonary and abdominal lesions due to D repens infection have been reported in endemic areas of Italy, France, and Greece.
Lung involvement, which is the most common of the noncutaneous/nonocular manifestations of dirofilariasis, is frequently misdiagnosed as a primary or metastatic lung tumor. [27]
Bacterial endosymbiont
Like many filarial worms, D repens harbors the bacterial endosymbiont Wolbachia, which has been implicated in filarial infections. In a study by Grandi et al, immunohistochemical staining of Wolbachia surface protein (WSP) on 14 skin nodules showed numerous bacteria within the intact worms and occasionally within surrounding inflammatory infiltrates. Serum samples from most of patients studied were positive for total immunoglobulin G (IgG) titers against WSP on enzyme-linked immunosorbent assay (ELISA), indicating a specific immune response to Wolbachia in patients with subcutaneous dirofilariasis. [28, 29]
Heartworm-associated respiratory disease results from the vascular and parenchymal inflammatory response associated with the arrival and death of Dimmitis worms into the distal pulmonary arteries. Some studies suggest the involvement of Wolbachia in the development of the inflammatory reaction and in the polarization of the host immune response against the parasite. [30] This parasite harbors intracellular Wolbachia, an endosymbiont bacteria. Fourteen seropositive cats to Wolbachia and 8 seronegative cats were put into the plethysmograph chamber and different respiratory variables were measured. Significant differences were found for bronchoconstriction index (pause [PAU]), suggesting Wolbachia seems to produce a greater acute inflammatoryresponse at bronchial, vascular, and parenchymal levels, worsening the state of bronchoreactivity associated with the presence of seropositivity to D immitis.
Immune response
Dirofilariasis is characterized by a dual Th1/Th2–type immune response. [31] Analysis of mRNA expression of cytokines and of serum antibody subisotopes in naturally infected dogs has demonstrated that microfilaremic infections associate to a predominant Th2-type response mainly directed against D immitis antigens, while a Th1-type response against the WSP predominates in amicrofilaremic infections. [32]
Among human dirofilariasis cases, exposed individuals who have not developed further pathology demonstrate production of Th2-mediated immunoglobulin E (IgE) antibodies directed against specific proteins from D immitis, [33] while a Th1-type response against WSP is detected in most patients with human pulmonary dirofilariasis (HPD), a pulmonary form of D immitis infection. [34]
Etiology
Dirofilariasis is a helmintic infection caused by parasites of the Dirofilaria genus (genus Dirofilaria, family Onchocercidae, which is included in the class of Nematodes). It is a common zoonotic disease affecting mostly dogs (lord hosts), other canines (wolves, coyotes), foxes, and cats.
The Dirofilaria species that most commonly cause human infection are D immitis (heartworm) and D repens. It is transmitted to humans through arthropods; mosquitoes are the vector for Dirofilaria. Infection with these nematodes, however, is independent of dog ownership, although residence in or travel to an area where canine dirofilariasis is endemic is almost universal among cases of human dirofilariasis.
Because some mosquito species that transmit dirofilariasis feed indiscriminately on numerous animal species and humans, the occurrence of D immitis and D repens infections in a canine population implies a risk of infection for the local feline and human populations. [34, 35]
Human disease caused by Dirofilaria species that infect other animals—including D tenuis (raccoon worm), D ursi (bear), D subdermata (porcupine), D lutrae (North American otter), D striata (wild American felines), and D spectans (Brazilian otter)—is occasionally reported in the medical literature.
D tenuis has been described as a cause of subcutaneous facial nodules and ophthalmic dirofilariasis in Florida, while an adult female D striata was removed from the orbit of a 6-year-old boy living in North Carolina, and D spectans was extracted from a digital artery lesion of a Brazilian man.
Human immunodeficiency predisposing to dirofilariasis
Initial theories suggested that the increased incidence of HPD found in elderly individuals (in contrast to the correspondingly low incidence of the condition in children) was the result of failing immunity. This theory has been disputed, however, as only 14% of adults diagnosed with HPD have a coexistent immune defect.
Dirofilariasis in wildlife
The presence of D immitis infection is becoming increasingly recognized in North American wildlife (ie, coyotes, foxes, wolves, black bears) that share environments with humans. This may become increasingly relevant in the future control of dirofilariasis, because prophylactic antifilarials can be administered to domestic canines to eradicate and control Dirofilaria in urban areas.
Wild canids
In the United States, the prevalence of D immitis in coyotes varies from 7% (Missouri) to 75% (Georgia) to 91% (northern California, United States). [36, 37, 38]
Two to 24 months after the release of captive red wolves, who had initially tested negative for microfilariae, into the Alligator River National Wildlife Refuge, in North Carolina, 14% of them tested positive for D immitis.
Fifty-six percent of dingoes (Australian wild dogs, or Canis familiaris dingo) in a tropical region of Australia’s Northern Territory were found to be infected with adult D immitis worms.
Foxes
In the United States, the prevalence of D immitis in foxes varies from 6% (Missouri) to 21% (South Carolina). [39]
The urban prevalence of D immitis in red foxes in Australia ranges from 6.4% (Melbourne) to 8.8% (Sydney). [40] In the Melbourne study, 2 known species of mosquito vector were also identified, increasing the risk of human infection. In Spain, 11% of red foxes in the Pyrenees region were found to be infected.
Raccoons and bears
In the United States, D tenuis was detected in 20% of wild raccoons trapped in southeast Georgia. [41]
D ursi has been detected in 0.2% of black bears in New Brunswick, Canada. [42]
Epidemiology
Prevalence in animals
D immitis
The prevalence of dirofilariasis in domestic dogs and cats varies by state and geographic region throughout the United States. All states except Nevada have reported D immitis infection in dogs, with the prevalence varying from low levels in some states (0.3%, 0.5%, and 1.2% in Colorado, Washington, and Montana, respectively) to as high as 40% in Florida and South Carolina. Of stray cats in Michigan, 3% were found to have adult D immitis worms at autopsy. In general, the infection is more prevalent in dogs than in cats, in which prophylaxis is not routinely recommended.
A similar variation in prevalence of D immitis in dogs and cats occurs elsewhere in the world, except Japan, where as many as 10% of stray cats are infected. [43] In Europe, the recorded prevalence rate of D immitis in dogs is as follows:
-
Greece - 5-11%
-
Barcelona, Spain - 13%
-
Northeastern Italy - 44-55%
-
Canary Islands - 59%
In other parts of the word, the prevalence of D immitis in dogs includes the following:
-
Brazil - Approximately 2% of dogs in Recife, Brazil, are infected with D immitis [47]
-
Taiwan - A prevalence of 55% was recorded in dogs of northern Taiwan
D repens
The prevalence of D repens in dogs is also substantial in endemic areas of southern Europe. The reported rates are as follows:
-
Greece - 7-22%
-
France - 20%
-
Italy - 29% in Sicily
-
Spain - 37-85% in some areas
The rate of dirofilariasis in dogs in Sri Lanka, another area endemic for D repens, is high, with rates of 30-60%.
Occurrence in the United States
The epidemiology of human dirofilariasis is related to the prevalence of canine dirofilariasis, the presence of suitable mosquito vectors, and human activities that lead to exposure.
D immitis causes most cases human dirofilariasis in the United States, with the prevalence of canine dirofilariasis having increased since the late 20th century. The geographic spread of the human disease has paralleled that of the dog population, although human dirofilariasis tends to be independent of dog ownership.
Most HPD cases have been found in the states adjacent to the Gulf of Mexico and the southern Atlantic states, where the prevalence of canine D immitis infection has been documented to be as high as 40%. The entire range of canine D immitis infection extends much further, however, existing along the Mississippi River Valley and into southern Canada. Canine dirofilariasis has been described in all of the lower 48 states except Nevada.
The first human case of dirofilariasis in the United States was described in a female cadaver in New Orleans in 1941. The first description of HPD causing pulmonary infarction was in 1961. Since then, at least 197 cases of HPD, presumably caused by D immitis, have been reported. Ten cases of D ursi infection, 3 cases of D tenuis subcutaneous infection, and isolated cases of D striata and possible D lutrae infection have also been reported.
International occurrence
The most commonly reported manifestation of human dirofilariasis worldwide is subcutaneous or ocular disease caused by D repens, with 800 case reports in the medical literature. D repens is an Old World parasite and has not been described in the Americas, Japan, or Australia.
At temperatures below 14°C, Dirofilaria stops developing, explaining the predominant occurrence of dirofilarial infections in warmer climatic zones. [48]
Dirofilariasis is endemic around the Mediterranean basin, [49] with Italy being the European country with the highest prevalence of human dirofilariasis (66%), followed by France (22%), Greece (8%), and Spain (4%). The great majority of cases reported in France have occurred below the 46th parallel. [50]
However, although most human cases of dirofilariasis have been diagnosed in areas where canine dirofilariasis has been endemic for many years, Dirofilaria nematodes are currently considered emerging agents of parasitic zoonoses in Europe. Currently, 45% of the total human population of Europe, as well as their domestic and companion animals, are exposed to the risk of vector-borne helminths (VBH) causing diseases. Canine dirofilarioses by D immitis and D repens are key examples of the success of VBH spreading into nonendemic areas. [51]
Climatic changes and an increase in the movement of the parasite’s reservoirs (mostly infected dogs) have caused an increase in the geographic range of Dirofilaria from the traditionally endemic/hyperendemic southern regions, and the risk of human infection has consequently increased as well. In the last several years, forecast models have predicted that current summer temperatures are sufficient to facilitate extrinsic incubation of Dirofilaria in many areas of Europe. Drepens is currently the filarial species most commonly reported as spreading from southern to northern areas. [52, 53, 54]
The global warming anticipated by the Intergovernmental Panel on Climate Change suggests that warm summers suitable for Dirofilaria transmission in Europe will become more common; if this eventuates, filarial infection may spread into previously infection-free areas. [48, 55]
Until 2006, only imported cases of canine and human disease had been described in northern Europe. Szénási et al, however, subsequently described the first autochthonous human case of D repens infection in Hungary, supporting the concept that dirofilariasis is an emerging zoonosis in Hungary and potentially elsewhere in Central Europe. [56] Autochthonous human dirofilariasis also exists in Ukraine, Russia, and Belarus, which have seen a dramatic increase in the number of reported cases. [57, 58] Most cases are reported in the southwestern regions of Russian Federation; however, the disease has spread into northern areas, changing its limit from latitude 45 N to 61 N in 2013. [59] A few cases have also been reported in Croatia and Slovakia. [60, 61] Endemic foci of D repens infection exist in Asia Minor, Central Asia, and Sri Lanka. [62]
Most cases of HPD have been detected in the United States and Japan, but the disease has also been described in many other countries on all inhabited continents, including South America (Brazil), Europe (France, Italy, Spain), Asia (Ukraine, Japan, India), Africa, and Australia. [63]
The incidence of ocular dirofilariasis is on the rise in several parts of India, particularly in Kerala. [6] Human exposure to D immitis larvae has also been reported in isolated communities of Colombian Indian tribes of the Amazon rainforest.
Overall, the volume of reported cases of dirofilariasis and the number of affected countries have been on the increase. In addition to the aforementioned climate changes, several other factors may be responsible for the increase, including greater medical awareness in more countries. Another factor may be increased travel to endemic countries; most described cases of dirofilariasis in central and northern Europe have been attributed to travel to endemic zones of southern Europe or the United States.
According to some suggestions, the true prevalence of human exposure to and disease caused by D immitis is underestimated, because canine infection is widespread throughout the world and most infected people are asymptomatic.
A seroprevalence study in Spain, where 33% of dogs are infected with D immitis, [64] revealed that 22% of humans were seropositive (immunoglobulin G [IgG], 5.8%; IgM, 3.5%; IgE, 12.6%). [65, 66] IgG seroconversion was most prevalent in people older than 60 years, whereas IgM seropositivity was most common in persons younger than 19 years. The level of IgE was also found to decrease with age. The authors concluded that repeated contact with D immitis in this endemic population was common and began at an early age.
Race-related demographics
All studies of HPD from the United States have reported a predominance of infection in people of European descent (as high as 95%). This probably indicates a selection bias, with more white Americans presenting for radiologic screening investigations than African Americans.
Sex-related demographics
The incidence and prevalence of human dirofilariasis shows a sex preference that depends on the infecting species, as follows:
-
D immitis infection: The reported male-to-female ratio of HPD is 2:1 in US patients
-
D repens infection: Women represent 55% of worldwide infections
Age-related demographics
D immitis infection
All reviews of HPD report that most affected adults are age 50-60 years. However, this may be explained by selection bias, as adults are more likely to undergo chest radiography for cancer screening or clinical pulmonary disease. HPD has been described in children as young as age 8 years. [49, 67]
D repens infection
This also occurs more commonly in adults, peaking in individuals aged 40-49 years. The exception is in Sri Lanka, where children younger than 9 years are most likely to be infected. The youngest infected individual reported there was aged 4 months. This pediatric predominance is attributed to the custom in Sri Lanka of allowing male toddlers to completely eschew clothing or to wear only upper garments. The theory is supported by the observation that most pediatric cases in Sri Lanka are localized to the scrotum, penis, and perineal regions of the body. [62]
Prognosis
In general, following diagnosis and resection of the affected tissue, the prognosis for patients with dirofilariasis is excellent.
Complications with dirofilariasis are rare. The most common ones are nonspecific respiratory symptoms or a small pulmonary infarct that brings the infection to medical attention. Intraocular, subconjunctival, and retro-orbital [68] infections have also been reported. [69, 70, 71] A single case report describes meningoencephalitis and aphasia complicating infection with D repens. [72] No recorded fatalities due directly to dirofilariasis have been reported in the medical literature.
The primary clinical significance of HPD is the almost invariable radiologic misdiagnosis of a primary or metastatic lung tumor, which usually leads to thoracotomy with open lung biopsy or wedge resection of the lung to obtain the correct diagnosis.
-
Plain chest radiographic appearance of pulmonary coin lesion secondary to Dirofilaria immitis infection in a man.
-
Adult Dirofilaria immitis worm extracted from the heart and pulmonary artery of a dog. Worms usually are 1-3 mm in diameter, and females may grow to as long as 30 cm.
-
Transverse section through an immature adult Dirofilaria immitis removed from the right chest wall of an 18-month-old child in Sydney, Australia. The large lateral chords and multilayered cuticle are typical of Dirofilaria. The smooth cuticle is a feature of D immitis.
-
Transverse section through a mature female Dirofilaria repens removed from the superomedial orbital rim of a 67-year-old man. The patient was infected in Corfu, Greece, 6 months prior to diagnosis in Sydney, Australia. Features characteristic of Dirofilaria are the arrangement of the longitudinal muscles and the multilayered cuticle, which is expanded in the region of the large lateral chords.
-
Higher-magnification image of the Dirofilaria repens displayed in the previous image. The nematode was removed from the eye of a 67-year-old man. The features characteristic of D repens are the longitudinal ridges on the cuticle; the ridges are 6-7 micrometers wide, spaced at 11- to 12-micrometer intervals.
-
Low-power view of a bulbar conjunctival biopsy specimen obtained from a 72-year-old man from Queensland, Australia, showing degenerate pieces of an immature Dirofilaria immitis worm in cross-sections and longitudinal sections with Masson stain.
-
Midpower view of bulbar conjunctival biopsy specimen showing degenerate pieces of an immature Dirofilaria immitis worm in cross-section and longitudinal section view with Masson stain.
-
High-power view of bulbar conjunctival biopsy specimen showing a cross-section of immature Dirofilaria immitis. The thin, smooth cuticle with internal, lateral, longitudinal ridges; the thin hypodermis; the large lateral cords; the well-developed coelomyarial muscles; and the ill-defined reproductive organs are diagnostic of an immature D immitis (hematoxylin and eosin stain).






