Background
An ostium primum atrial septal defect (ASD), as seen in the image below, is located in the most anterior and inferior aspect of the atrial septum. It is the simplest form of atrioventricular (AV) canal or AV septal defect. These defects are often associated with trisomy 21.
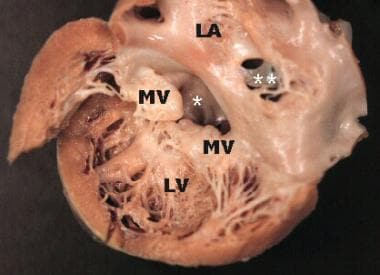 Gross pathology specimen viewed from the opened left atrium and left ventricle, demonstrating a partial atrioventricular (AV) septal defect. An ostium primum atrial septal defect (ASD) marked by an asterisk (*) is visualized in the inferior aspect of the interatrial septum. An ostium secundum ASD marked by two asterisks (**) is also noted. The mitral valve is cleft and the leaflets are thickened and rolled, suggestive of chronic mitral regurgitation. LA = left atrium, LV = left ventricle, and MV = mitral valve.
Gross pathology specimen viewed from the opened left atrium and left ventricle, demonstrating a partial atrioventricular (AV) septal defect. An ostium primum atrial septal defect (ASD) marked by an asterisk (*) is visualized in the inferior aspect of the interatrial septum. An ostium secundum ASD marked by two asterisks (**) is also noted. The mitral valve is cleft and the leaflets are thickened and rolled, suggestive of chronic mitral regurgitation. LA = left atrium, LV = left ventricle, and MV = mitral valve.
During fetal development, the rudimentary atrium is divided by the septum primum, except for an anterior and inferior space that is the ostium primum. The ostium primum is sealed by fusion of the superior and inferior endocardial cushions around 5 weeks' gestation. Failure to do so results in an ostium primum ASD. Observations by Anderson and colleagues suggest that failure of growth of the vestibular spine to complete atrial septation may result in the ostium primum atrial defect. [1]
The endocardial cushions also contribute to the complete formation of two separate AV valves and the inlet interventricular septum. For this reason, ostium primum ASDs are commonly associated with malformations of these structures.
Ostium primum ASDs are most commonly seen with a cleft in the anterior leaflet of the mitral valve, but the mitral valve cleft may occur in isolation. This is sometimes termed a partial AV canal defect or a partial AV septal defect. In this case, a five-leaflet AV valve is arranged so that separate right and left components (a tricuspid valve and a mitral valve) are present. The leaflets connect to each other and then adhere to the crest of the interventricular septum. This results in shunting at the atrial level with no ventricular level shunting. Generally, a commissure is observed between the left superior and inferior bridging leaflets because of abnormal fusion of the left tubercle of the superior and inferior cushions, which results in a cleft in the anterior leaflet of the mitral valve.
For patient education resources, see Heart Health Center, as well as Palpitations.
Pathophysiology
Shunting is predominantly left-to-right in the absence of pulmonary vascular disease or significant right ventricular outflow tract obstruction. This results in volume overload of the right atrium and ventricle and pulmonary overcirculation. If the mitral valve cleft causes significant mitral regurgitation, the left side of the heart also becomes volume overloaded. A left ventricle-to-right atrium shunt can be present, which further overloads both the right and left hearts.
Etiology
The most common association of an ostium primum atrial septal defect (ASD) is genetic, associated with trisomy 21. Well-described associations have also been reported with Holt-Oram syndrome, Noonan syndrome, and Ellis-van Creveld syndrome, among others. [2] In children with normal chromosomes, however, the cause remains unknown. Research into the molecular genetic basis for atrioventricular (AV) canal and AV septal defects is ongoing.
A study by Rana et al implicated the TBX1 gene in the development of ostium primum ASDs, among other congenital heart defects. According to these investigators, TBX1 -null embryos are impaired in the ability of second heart field cells (multipotent cardiovascular progenitor cells) to be added to the venous pole of the heart, causing ostium primum defects, as well as abnormal development of the dorsal mesenchymal protrusion. [3]
Epidemiology
United States statistics
Ostium primum atrial septal defects (ASDs) are most commonly associated with Down syndrome (trisomy 21). The incidence of trisomy 21 is 1 per 800 live births, with an increased prevalence observed in children born to older mothers. Note the following:
-
The overall risk of congenital heart disease in patients with Down syndrome is 40-50%. Approximately 65% of those affected have some form of atrioventricular (AV) septal defect.
-
The inherited risk for children of parents who have an AV septal defect is reported as 9-14%.
Sex- and age-related demographics
The male-to-female ratio is 1:1.
Patients with ostium primum ASD typically present at a young age. Patients very rarely reach adulthood without surgical correction. [4, 5, 6]
Patients with smaller defects and little or no mitral regurgitation may present at any age with a murmur and/or an abnormal electrocardiogram. Those with more severe mitral regurgitation typically present with congestive heart failure in the first 1-2 years of life.
Prognosis
Pediatric patients with small left-to-right shunts and no significant mitral regurgitation who have not undergone surgery are at relatively low risk for complications. In these patients, survival through adulthood is expected, but complications can develop as age advances. [7] Untreated patients with large shunts and/or significant mitral regurgitation are at significant risk of morbidity and mortality. Death, arrhythmia, heart block, refractory heart failure, and advanced pulmonary vascular disease are the most common complications and tend to increase with advancing age. Pulmonary vascular obstructive disease may develop in a subset of patients—and those with Down syndrome are at the highest risk. [8] The prognosis is guarded, and morbidity and mortality are high regardless of therapy.
Surgical repair generally improves life expectancy and alters the natural course of the disease. Investigators at the Hospital for Sick Children in Toronto evaluated the long-term outcome of 180 children with ostium primum atrial septal defects (ASDs) from 1982 to 1996 and found that age and preoperative moderate-to-severe left atrioventricular (AV) valve regurgitation were predictors of reoperation, and age at repair younger than 1 year was a predictor of death. [9] Three patients (1.6%) suffered early mortality, two of whom were infants. Another 17 patients (9%) underwent reoperation (five infants); five patients underwent reoperation for subaortic obstruction, and 12 for left AV valve regurgitation (one required valve replacement, 11 were repaired). Actuarial survival was 98% at 10 years with no late deaths. [9]
A study from Oregon Health Sciences University that assessed 38 consecutive patients aged 3-58 months who underwent surgical correction for partial AV septal defect between 1981 and 1997 concluded that an aggressive approach to early operative intervention is safe and effective, as well as provides good long-term results. [10] Nearly all the patients (92%) underwent closure of the mitral cleft, with 28% of these patients also requiring mitral annuloplasty. Early 30-day mortality was 7.9%. At follow-up up to 14 years, late mitral regurgitation was present in 0.9% of the patients, and there was only one late reoperation. At last follow-up, 13% of the patients were symptomatic. [10]
Data from Italian investigators supported the conclusions of Oregon Health Sciences University researchers. Michielon et al documented 93.5% (±2%) freedom from reoperation at 12.3 years for partial AV septal defects. [11] Reintervention was highest in patients with preoperative AV valve regurgitation and a double orifice left AV valve but was statistically lower for patients who underwent early repair using a bifoliate approach. Good results were attributed to the prevention of progressive mitral annular dilatation. [11]
The Mayo Clinic reviewed the need for reoperation over a 45-year period (1962-2006) and found that when reoperation was required, overall late survival was significantly reduced. [12] Ninety-six patients underwent reoperation (median interval, 10 years), with a median age at first reoperation of 26 years (range, 10 months to 71 years). Indications included left AV valve (LAVV) regurgitation in 67% of patients, subaortic stenosis in 25% of patients, right AV valve regurgitation in 22% of patients, residual ASD in 11% of patients, and other indications in 6%. Of the five early deaths, three occurred prior to 1983. No significant difference was noted in 20-year survival after LAVV repair or replacement (69% vs 55%, P = 0.20). At last follow-up (median, 5.2 years; max, 34 years), 81 of 89 late survivors had New York Heart Association (NYHA) functional class I or II. [12]
The Mayo Clinic also previously reviewed the experience in adults of 31 patients aged 40-71 years at the time of repair at their institution from 1958 to 1990 [13] ; 23 had repair of the mitral cleft, two required mitral valve replacements, and six warranted mitral reoperation. Early mortality was 6%; 19 patients were followed for a mean of 14 years, with 14 reporting a sustained postoperative improvement. [13]
The Pediatric Heart Network evaluated the need for reoperation for partial versus other forms of ASDs in 215 patients from seven North American centers and found that the subtype of AV septal defect was significantly associated with preoperative patient characteristics and clinical status as well as influenced the age at repair. [14] Patients were subtyped as partial (n = 60), transitional (n = 27), complete (n = 120), and canal-type ventricular septal defect (VSD) (n = 8). The highest preoperative prevalence of moderate or severe LAVV regurgitation occurred in those with transitional ASD (P = 0.01). [14]
Significant postoperative LAVV regurgitation was the most common sequela (similar prevalence across all centers at 6 months). [14] At 6-month follow-up, older age at repair was an independent predictor of moderate or severe LAVV regurgitation (P = 0.02) but not annuloplasty, subtype, or center (P >0.4). After accounting for age at repair, there was no association seen between AV septal defect subtype and postoperative LAVV regurgitation severity or growth failure at 6 months. [14] Annuloplasty failed to decrease the postoperative prevalence of moderate or severe LAVV regurgitation at 6 months
Morbidity/mortality
The presence and degree of associated mitral regurgitation and/or left ventricle-to-right atrium shunting generally determine the symptoms.
Patients with either no cleft or a cleft with a mild degree of mitral regurgitation are often asymptomatic. Patients typically are referred for evaluation of a heart murmur in childhood and generally survive well into adulthood. However, adults who have not had the condition repaired often become symptomatic from congestive heart failure (CHF) by age 45 years. [15] Rarely, patients are reported to present in the seventh decade of life. [16] Dyspnea on exertion and fatigue are the usual complaints in adults, as are palpitations secondary to atrial fibrillation or flutter.
Those with more severe mitral regurgitation or left ventricle-to-right atrium shunting often present in the first 2 years of life. Mortality has been reported to be as high as 30% in this subpopulation in the first year of life.
Although relatively rare, pulmonary vascular obstructive disease may occur in patients with long-standing substantial shunts and significant mitral regurgitation.
As noted earlier, children with trisomy 21 are at higher risk than the general population of developing pulmonary vascular obstructive disease at a younger age. Potential reasons for this include chronic upper airway disease, tonsillar and adenoid hypertrophy, and inadequate alveolarization of the terminal bronchioles, leading to a decreased surface area of the vascular bed.
Complications
Infective endocarditis remains both a preoperative and a postoperative complication. In a study from Oregon Health Sciences University, the 30-year postoperative incidence of infective endocarditis was 2.8% among patients with ostium primum ASDs. [17]
-
Electrocardiogram from a patient with a partial atrioventricular septal defect. The PR interval is mildly prolonged. Left axis deviation with Q waves in leads I and aVL are present, consistent with a counterclockwise loop in the frontal plane. Right atrial enlargement and an rsR' pattern in the right chest leads are also noted.
-
Two-dimensional, apical, four-chamber echocardiogram of a partial atrioventricular (AV) septal defect. The asterisk (*) delineates an area of dropout in the inferior atrial septum at the site of the primum atrial septal defect. The AV valves are separate but aligned at the same horizontal level, consistent with a two-orifice common AV valve. In systole, the medial leaflets of the right- and left-sided AV valves demonstrate attachments to the crest of the interventricular septum, allowing no ventricular level shunting. LA = left atrium, LV = left ventricle, RA = right atrium, and RV = right ventricle.
-
Gross pathology specimen viewed from the opened left atrium and left ventricle, demonstrating a partial atrioventricular (AV) septal defect. An ostium primum atrial septal defect (ASD) marked by an asterisk (*) is visualized in the inferior aspect of the interatrial septum. An ostium secundum ASD marked by two asterisks (**) is also noted. The mitral valve is cleft and the leaflets are thickened and rolled, suggestive of chronic mitral regurgitation. LA = left atrium, LV = left ventricle, and MV = mitral valve.







